Pipeline
Barinthus Biotherapeutics is undertaking clinical studies for HBV and HPV therapeutics.
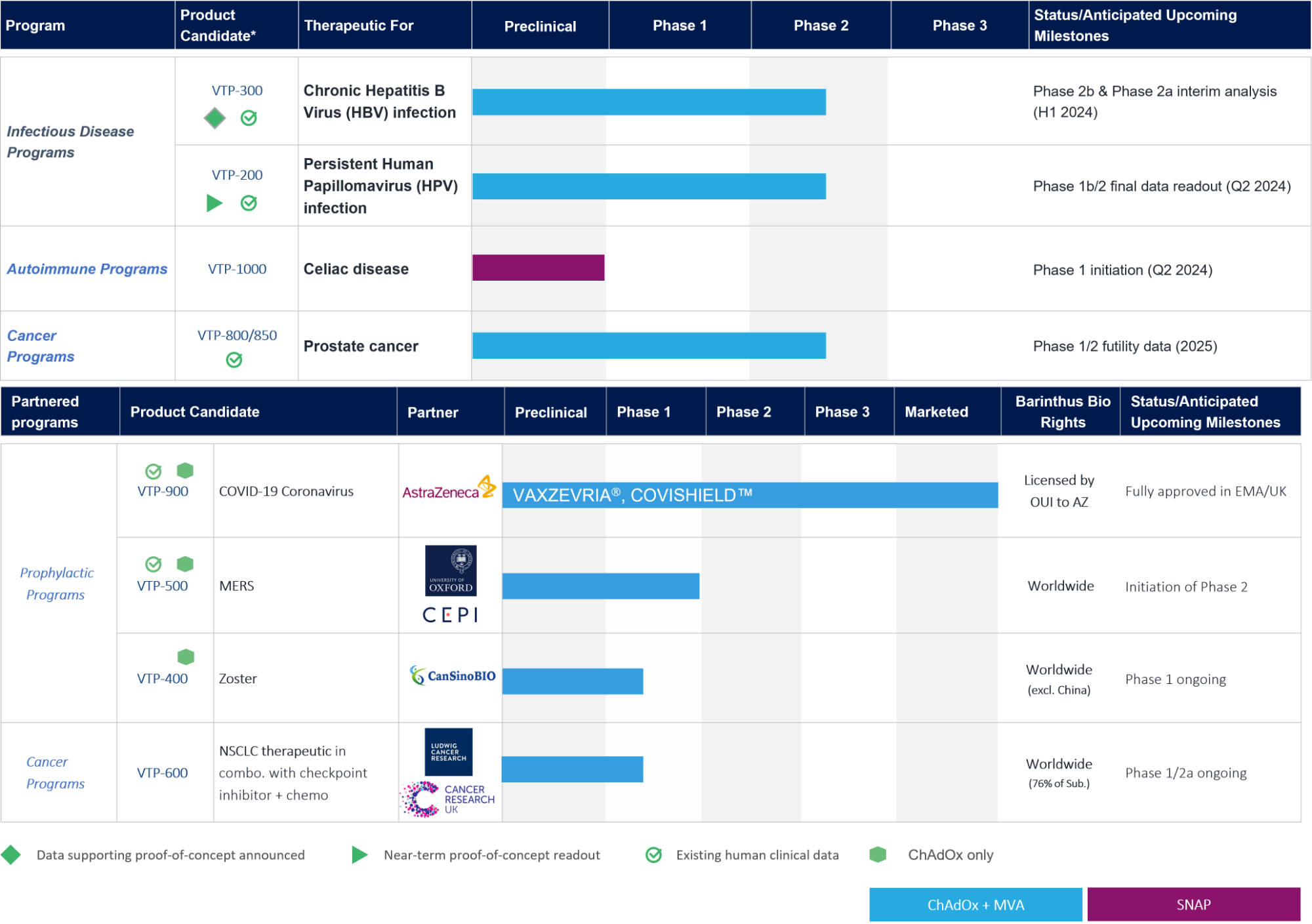
These are estimated timelines only and our pipeline may be subject to change.
Updated: 5 January 2024

These are estimated timelines only and our pipeline may be subject to change.
Updated: 5 January 2024